Summary of our research
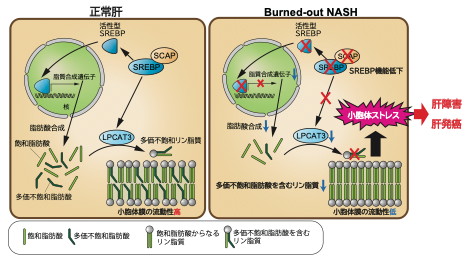
Enhanced de novo lipogenesis mediated by sterol regulatory element-binding proteins (SREBPs) is thought to be involved in nonalcoholic steatohepatitis (NASH) pathogenesis. In this study, we assessed the impact of SREBP inhibition on NASH and liver cancer development in murine models. Unexpectedly, SREBP inhibition via deletion of the SREBP cleavage-activating protein (SCAP) in the liver exacerbated liver injury, fibrosis, and carcinogenesis, despite markedly reduced hepatic steatosis. These phenotypes were ameliorated by restoring SREBP function. Transcriptome and lipidome analyses revealed that SCAP-SREBP pathway inhibition altered the fatty acid (FA) composition of phosphatidylcholines due to both impaired FA synthesis and disorganized FA incorporation into phosphatidylcholine via lysophosphatidylcholine acyltransferase 3 (LPCAT3) downregulation, which led to endoplasmic reticulum (ER) stress and hepatocyte injury. Supplementation of phosphatidylcholines significantly improved liver injury and ER stress induced by SCAP deletion. The activity of SCAP-SREBP-LPCAT3 axis was found inversely associated with liver fibrosis severity in human NASH. SREBP inhibition also cooperated with impaired autophagy to trigger liver injury. Thus, excessively strong and broad lipogenesis inhibition was counterproductive for NASH therapy, which will have important clinical implications in NASH treatment.
Researcher information

NAKAGAWA Hayato
Professor and Chairman, Department of Gastroenterology and Hepatology, Mie University
Specialized area:
Gastroenterology, Hepatology, Oncology
Current research field:
Molecular mechanisms of hepatobiliary injury and carcinogenesis